About Endo Advantage™
Endo Advantage™ provides a single point of contact for healthcare professionals (HCP) and patients to help support patient access from start to finish.

Once the decision to prescribe XIAFLEX® is made, acquisition services through Endo Advantage™ can assist you and your patients with:

INSURANCE
BENEFITS
INVESTIGATION
PRIOR
AUTHORIZATION

PATIENT FINANCIAL
ASSISTANCE
for those who qualify
Acquiring XIAFLEX® : Quick tips for your practice
Learn how Endo can support your office staff to obtain
Benefits Investigation
Endo Advantage™ can conduct a benefits investigation for your patient
Download these forms and resources to assist with access and reimbursement for XIAFLEX®
- Office Administration Handbook
Reference this guide when planning and preparing for reimbursement of
XIAFLEX® . - Copay Assistance Proof of Expense Form
Have patients fill out this form to request copay assistance for
XIAFLEX® . - Benefits Investigation Form
Use this form to initiate benefits investigation.
- Help Guide: Benefits Investigation Form
Reference this guide if you have questions when completing the Benefits Investigation Form.
- Help Guide: Patient Benefits
Reference this guide for an explanation of a patient’s Benefits Investigation Results Form.
- Coding for
XIAFLEX® and Related ProceduresSelect the billing codes that most accurately describe the services provided.
- Helpline Fax Cover Sheet
Healthcare professionals/office staff can use this sheet to request assistance from Endo Advantage™.
- Patient Assistance Program Application
Have patients fill out this form to apply for the Patient Assistance Program.
- Letter of Medical Necessity
Sample letter for you to reference when drafting your request for coverage of injections for treatment.
- Product Acquisition Information
Contact information for a specialty distributor and specialty pharmacy.
- Chart Documentation Guide
Reference this sheet when charting for a patient receiving
XIAFLEX® . - Claim Form (with and without wastage)
Sample form for you to reference when submitting an insurance claim.
-
XIAFLEX® Rebate ProgramReference this sheet for information about rebates available for
XIAFLEX® . - Patient Assistance Product Request Form
Healthcare professionals can complete this form on behalf of appropriate patients to order
XIAFLEX® .
Acquiring XIAFLEX®
Once REMS-certified, you can order XIAFLEX® through 1 of 3 channels
Only REMS-certified physicians and healthcare sites can acquire
SPECIALTY DISTRIBUTOR
Purchase the product from our Specialty Distributor (SD) and bill the patient’s insurance for both
SD CONTACT INFORMATION
Besse Medical
1-800-543-2111 (phone)
1-800-543-8695 (fax)
www.besse.com
SPECIALTY PHARMACY PROVIDER
The Specialty Pharmacy Provider (SPP) will fill a patient-specific prescription for
SPP CONTACT INFORMATION
CVS Specialty
1-844-343-4221 (phone)
1-877-733-3194 (fax)
www.cvsspecialty.com
HOSPITAL-AFFILIATED HCP PRACTICES
HCPs can acquire
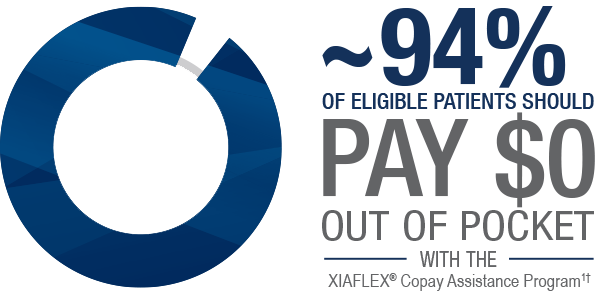
Patients can save with the Copay Assistance Program
With the XIAFLEX® Copay Assistance Program:
Eligible patients may save on their out-of-pocket costs for each vial of
Restrictions for patients apply. See restrictions, terms and conditions below.*
Patient eligibility
Patients qualify for assistance under this program if:
- They are receiving or have received
XIAFLEX® for an approved indication and in a manner consistent with the instructions for administration ofXIAFLEX® - They are uninsured or have insurance that is not provided by Medicare, Medicare Prescription Drug Benefit plans, Medicare Advantage, Veterans Affairs (VA), Medicaid, or similar federal or state programs, and this program is not otherwise prohibited by law
- They are 18 years of age or older
- They have paid or are obligated to pay out-of-pocket costs for a prescription of
XIAFLEX®
No other purchase is necessary to receive this offer.
For more information, call 1-800-743-2382.
By accepting this offer, you agree to report the value received under this offer to any health insurer or other third party paying for any part of your
XIAFLEX® prescription if you are required to do so by benefit terms, contract, or law.This offer is not valid for prescriptions reimbursed in whole or in part by Medicare, Medicare Prescription Drug Benefit plans, Medicare Advantage, VA, Medicaid, or similar federal or state programs, or where otherwise prohibited by law.
By accepting this offer, you agree that Endo Pharmaceuticals Inc. or those working on its behalf may contact your doctor to verify information about treatment that is relevant to verifying your eligibility for this offer.
This offer is only valid for doses of
XIAFLEX® administered in the US.This offer is valid for the out-of-pocket cost for a dose of
XIAFLEX® only. Offer is not valid for any other products or other out-of-pocket costs (for example, office visit charges, office visit copays, or injection/administration costs), even if those costs are associated with the administration of a dose ofXIAFLEX® .This offer is valid only if you have not used this program within the last 30 days.
The selling, purchasing, trading, or counterfeiting of this offer is prohibited.
Endo Pharmaceuticals Inc. reserves the right to rescind, revoke, or amend this offer without notice.
By participating, you understand and agree to comply with the terms and conditions of this offer as set forth above.
†Most eligible patients with commercial insurance plans should pay a $0 copay for




