Peyronie’s Disease
Helping patients find answersScreening considerations for Peyronie’s disease (PD)
Ask your patients about erectile curvature, because they may not ask you.1
Clinicians should engage in a diagnostic process to document the signs and symptoms that characterize Peyronie’s disease. The minimum requirements for this examination are a careful history and a physical exam of the genitalia (to assess for palpable abnormalities of the penis).2
- Depression3
- Erectile dysfunction4
- Hypogonadism5
- Type 2 diabetes mellitus6
Peyronie’s disease is a distinct condition. Treatment of Peyronie’s disease does not cover or imply treatment of these conditions.
When screening, take into consideration your patient’s Bother as well
What is the Bother domain score?
Clinical trials for XIAFLEX evaluated the Bother domain score, which is a composite of the following patient-reported items: concern about erection pain, erection appearance, and impact of Peyronie’s disease on intercourse and on frequency of intercourse.7,8
Patients with erectile curvature as low as 30 degrees may have similar Bother to patients with higher degrees of curvature8

Why many men may go undiagnosed
Percentage of men who visited an HCP for penile symptoms9:
received a PD
diagnosis
received no
treatment
were advised to
“wait and see”
were diagnosed with
erectile dysfunction
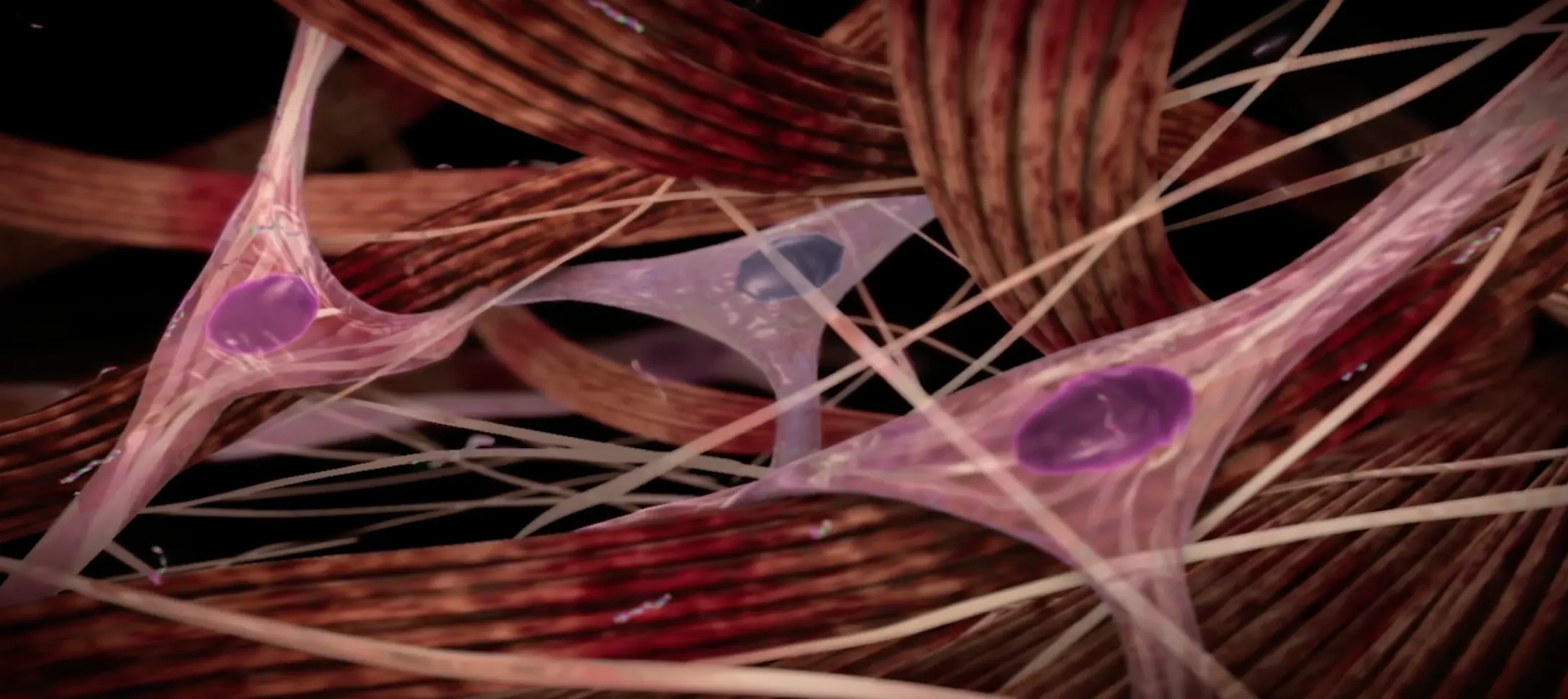
Understanding what causes Peyronie’s disease
Peyronie’s disease is a localized connective tissue disorder that affects the tunica albuginea of the penis.9-11
It is thought to occur after injury or repetitive microtrauma9-11
Abnormal scar tissue develops into a plaque under the skin of the penis, causing erectile curvature9-11
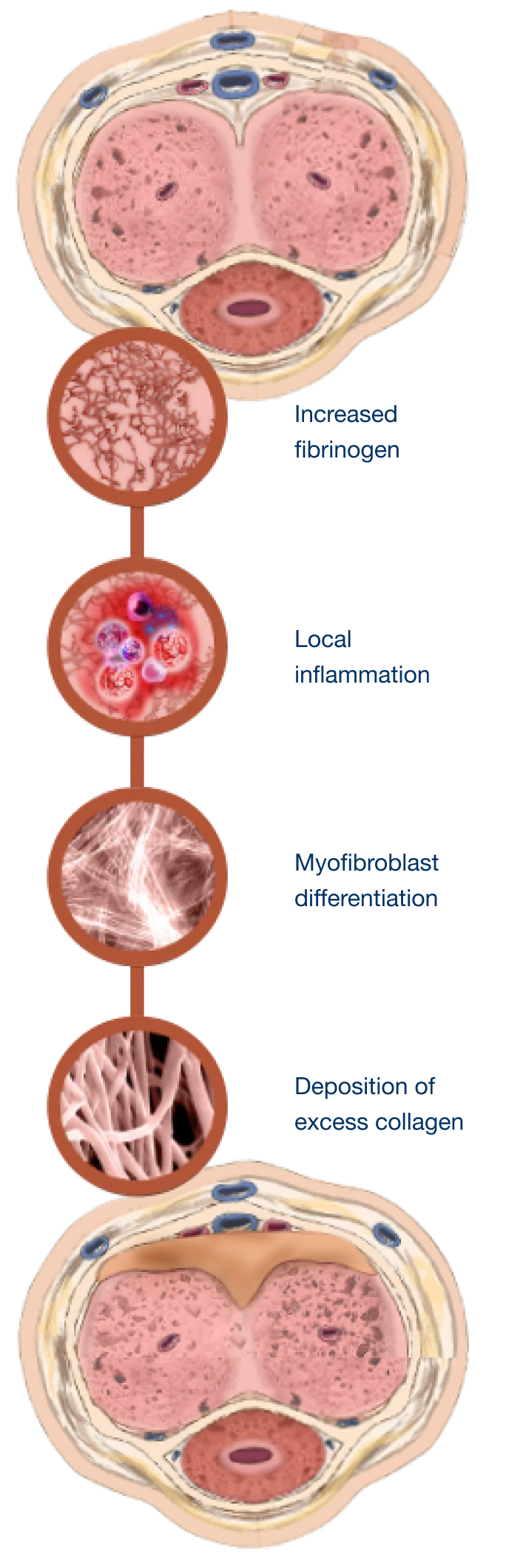
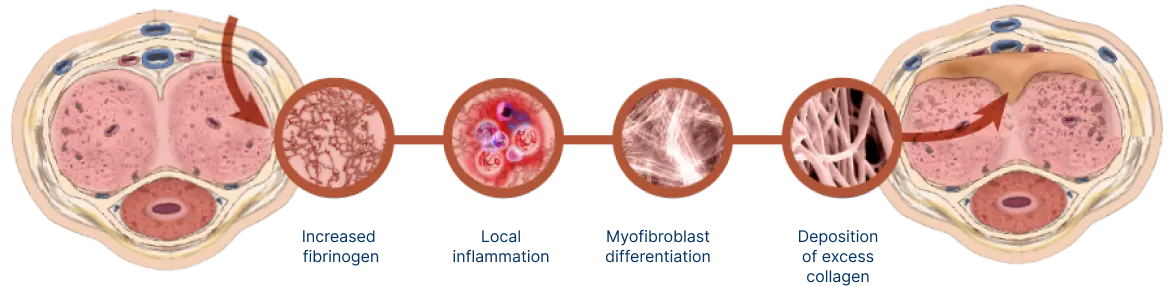
Mechanism for Peyronie’s disease
Watch and learn about the pathologic wound healing that is characteristic of Peyronie’s disease.
Important Safety Information
WARNING: CORPORAL RUPTURE (PENILE FRACTURE) OR OTHER SERIOUS PENILE INJURY IN THE TREATMENT OF PEYRONIE’S DISEASE
Corporal rupture (penile fracture) was reported as an adverse reaction in 5 of 1044 (0.5%) XIAFLEX‑treated patients in clinical studies. In other XIAFLEX‑treated patients (9 of 1044; 0.9%), a combination of penile ecchymoses or hematoma, sudden penile detumescence, and/or a penile "popping" sound or sensation was reported, and in these cases, a diagnosis of corporal rupture cannot be excluded. Severe penile hematoma was also reported as an adverse reaction in 39 of 1044 (3.7%) XIAFLEX‑treated patients.
Signs or symptoms that may reflect serious penile injury should be promptly evaluated to assess for corporal rupture or severe penile hematoma which may require surgical intervention.
Because of the risks of corporal rupture or other serious penile injury, XIAFLEX is available for the treatment of Peyronie’s disease only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the XIAFLEX REMS Program.
- Contraindications: XIAFLEX is contraindicated in the treatment of Peyronie’s plaques that involve the penile urethra due to potential risk to this structure and in patients with a history of hypersensitivity to XIAFLEX or to collagenase used in any other therapeutic application or application method
- Corporal Rupture or Other Serious Injury to the Penis: Injection of XIAFLEX into collagen-containing structures such as the corpora cavernosa of the penis may result in damage to those structures and possible injury such as corporal rupture (penile fracture). Therefore, XIAFLEX should be injected only into the Peyronie’s plaque and care should be taken to avoid injecting into the urethra, nerves, blood vessels, corpora cavernosa or other collagen-containing structures of the penis. Cases of localized skin and soft tissue necrosis occurring as sequelae of penile hematoma, some requiring surgical intervention, have been reported post-marketing
- Hypersensitivity Reactions, Including Anaphylaxis: In the double-blind, placebo-controlled portions of the clinical trials in Peyronie’s disease, a greater proportion of XIAFLEX-treated patients (4%) compared to placebo-treated patients (1%) had localized pruritus after up to 4 treatment cycles (involving up to 8 XIAFLEX injection procedures). The incidence of XIAFLEX-associated pruritus was similar after each injection regardless of the number of injections administered
- Because XIAFLEX contains foreign proteins, severe allergic reactions to XIAFLEX can occur. Anaphylaxis was reported in a post-marketing clinical trial in one patient who had previous exposure to XIAFLEX for the treatment of Dupuytren’s contracture. Healthcare providers should be prepared to address severe allergic reactions following XIAFLEX injections. The safety of more than one treatment course of XIAFLEX is not known
- Risk of Bleeding in Patients with Abnormal Coagulation: In the XIAFLEX controlled trials in Peyronie’s disease, 65.5% of XIAFLEX-treated patients developed penile hematoma, and 14.5% developed penile ecchymosis. Patients with abnormal coagulation (except for patients taking low-dose aspirin, eg, up to 150 mg per day) were excluded from participating in these studies. Therefore, the efficacy and safety of XIAFLEX in patients receiving anticoagulant medications (other than low-dose aspirin, eg, up to 150 mg per day) within 7 days prior to XIAFLEX administration is not known. In addition, it is recommended to avoid use of XIAFLEX in patients with coagulation disorders, including patients receiving concomitant anticoagulants (except for low-dose aspirin)
- Acute Post-Injection Back Pain Reactions: Post-marketing reports of acute lower back pain reactions, sometimes accompanied by radiation to the lower extremities, chest and arms, muscle spasms, chest pain, paresthesias, headache, and dyspnea, have been received by patients treated with XIAFLEX for Peyronie’s disease. These events can be mild to severe in intensity. The events typically lasted for 15 minutes and typically did not require intervention. Administer the smallest number of treatment cycles necessary to treat the patient’s curvature deformity
- Syncope and Presyncope: Most, but not all cases of syncope and presyncope in patients with Peyronie’s disease, occurred in association with post-injection penile pain and hematoma, penile pain with spontaneous erections, and pain during micturition. These potential triggers suggest a vasovagal mechanism. Make patients aware of the potential symptoms that could trigger syncope and presyncope after treatment with XIAFLEX.
If presyncopal symptoms occur, patients should remain recumbent until symptoms resolve. Syncope may be associated with bodily injuries, including concussion, head abrasion, and other accidental injuries
- In the XIAFLEX clinical trials for Peyronie’s disease, the most frequently reported adverse drug reactions (≥25%) and at an incidence greater than placebo included: penile hematoma, penile swelling, and penile pain.
- Acute post-injection lower back pain reactions have occurred in close temporal proximity to XIAFLEX treatments
- Cases of localized skin and soft tissue necrosis events as sequelae of penile hematoma, some of which required surgical intervention
- Syncope and presyncope have been reported in men treated with XIAFLEX for Peyronie’s disease. Most, but not all cases occurred in the immediate treatment period or within 1-2 days following injection. Bodily injuries associated with the syncopal events have been reported
References: 1. Hellstrom WJ. History, epidemiology, and clinical presentation of Peyronie’s disease. Int J Impot Res. 2003;15(Suppl 5):S91-S92.

